Building the Missing Layer of Precision Medicine: Why Patients Hold the Key to the Next Era of Healthcare
October 30, 2025

If you’ve ever watched someone you love cycle through treatments that don’t work, you know how powerless it feels. The scans are clear. The tests are fine. The doctor is kind. And yet, the person you love still suffers.
That experience is far more common than most people realise. Chronic conditions now account for the majority of the world’s disease burden, and in countries like the US and Australia roughly half of adults live with at least one long-term condition [1][2][3]. In the United States alone, six in ten adults have a chronic disease, and four in ten have two or more [2].
For millions of these people, science still can’t explain why one treatment works brilliantly for some and not at all for others. Nearly two decades after the Human Genome Project, medicine can map the code of life but still struggles to understand what happens inside most patients’ lives. Sequencing is getting cheaper. Algorithms are powerful. Yet the central promise of precision medicine - tailoring care to the individual - remains out of reach for most of the world.
Medicine has learned to read our DNA and biomarkers, but not yet our day-to-day. That is the next frontier.
The Promise and the Problem of Precision Medicine
Precision medicine is meant to tailor care to the individual: your biology, your environment, and your lived experience. It is how healthcare moves beyond averages.
Yet as The BMJ noted in 2023, the bottleneck in precision medicine is not sequencing capacity but contextual data about people’s lives [4]. We have learned how to read the genome but not the human story that shapes how biology unfolds in real life.
Health records capture moments, not motion. They freeze time instead of following change. Electronic health records, designed primarily for billing rather than biology, were never built to capture how people actually live, respond and recover [5][6].
The result is a system that knows how to measure many diseases but not recovery, how to test drugs but not the daily patterns that make them work or fail. Without that context, even the most advanced AI or genomic insight stops short of true precision.
To realise that promise, medicine needs continuous, real-world data that connects biology with lived experience, at scale and with consent.
AI Can’t Answer What Science Doesn’t Know
AI is transforming how we interpret health information. DeepMind’s AlphaFold has predicted over 200 million protein structures, solving what many scientists once called a 50-year grand challenge in biology [7]. Generative models are now designing new molecules in months instead of years. Insilico Medicine entered human clinical trials in 2023 with an AI-designed molecule for idiopathic pulmonary fibrosis, compressing a process that once took a decade into less than four [8].
AI systems are also matching or outperforming clinicians in certain diagnostic imaging tasks, from diabetic-retinopathy screening to cardiac-risk prediction, according to recent reviews in npj Digital Medicine [9]. These breakthroughs mark an extraordinary acceleration of discovery.
But AI can only build on what it knows. It learns from the sum of human science and creativity. Where evidence is thin, AI cannot create answers. When knowledge is missing, it often tries anyway, sometimes convincingly but dangerously so. Researchers warn that large language models can produce confident but incorrect medical answers, creating an illusion of knowledge that risks widening the very gaps we hope AI will close [10][11][12].
This is the paradox of modern medicine: the tools are extraordinary, but the foundation is incomplete. The conditions that affect billions, such as autoimmune disease, long COVID, chronic pain, mental-health conditions and reproductive disorders remain poorly understood because they are difficult to measure in the real world.
And the people most affected by those gaps are often those whose experiences medicine has historically ignored. Women, people of colour, older adults, people with disabilities, and individuals with overlapping conditions have all been consistently under-represented in research. Despite comprising more than half the global population, women still make up fewer than 30 percent of participants in early-phase trials [13]. A 2025 umbrella review in the International Journal for Equity in Health found that ethnic-minority participants remain significantly under-represented across health studies worldwide [14]. When science overlooks these groups, algorithms inherit those blind spots and inequities deepen.
Until we fill those evidence gaps with real-world, inclusive data, AI will keep reflecting the limits of what we know instead of expanding them.
Building the Missing Layer
We started Human Health to change that. To close the evidence gap for people and conditions that have long been under-researched. Our mission is to build the missing layer of precision medicine, grounded in lived experience and inclusive by design.
If you or a loved one has an autoimmune disease, a reproductive condition, or a mental health disorder, you know how little a blood test or DNA sequence can tell you. These conditions unfold over time, in energy levels, symptom and pain patterns, mood shifts and sleep changes that rarely show up in lab results. To make care precise, medicine first needs to understand those lived patterns.
And for most people, there is no single miracle drug. Improvement often comes from combinations of interventions: medication, nutrition, rest, therapy, exercise, lifestyle, and environmental changes that interact in ways formal trials rarely capture. Traditional research isolates one variable at a time, but life and often recovery does not work that way. To truly understand what helps, we need to measure interventions as well as outcomes, continuously and agnostically.
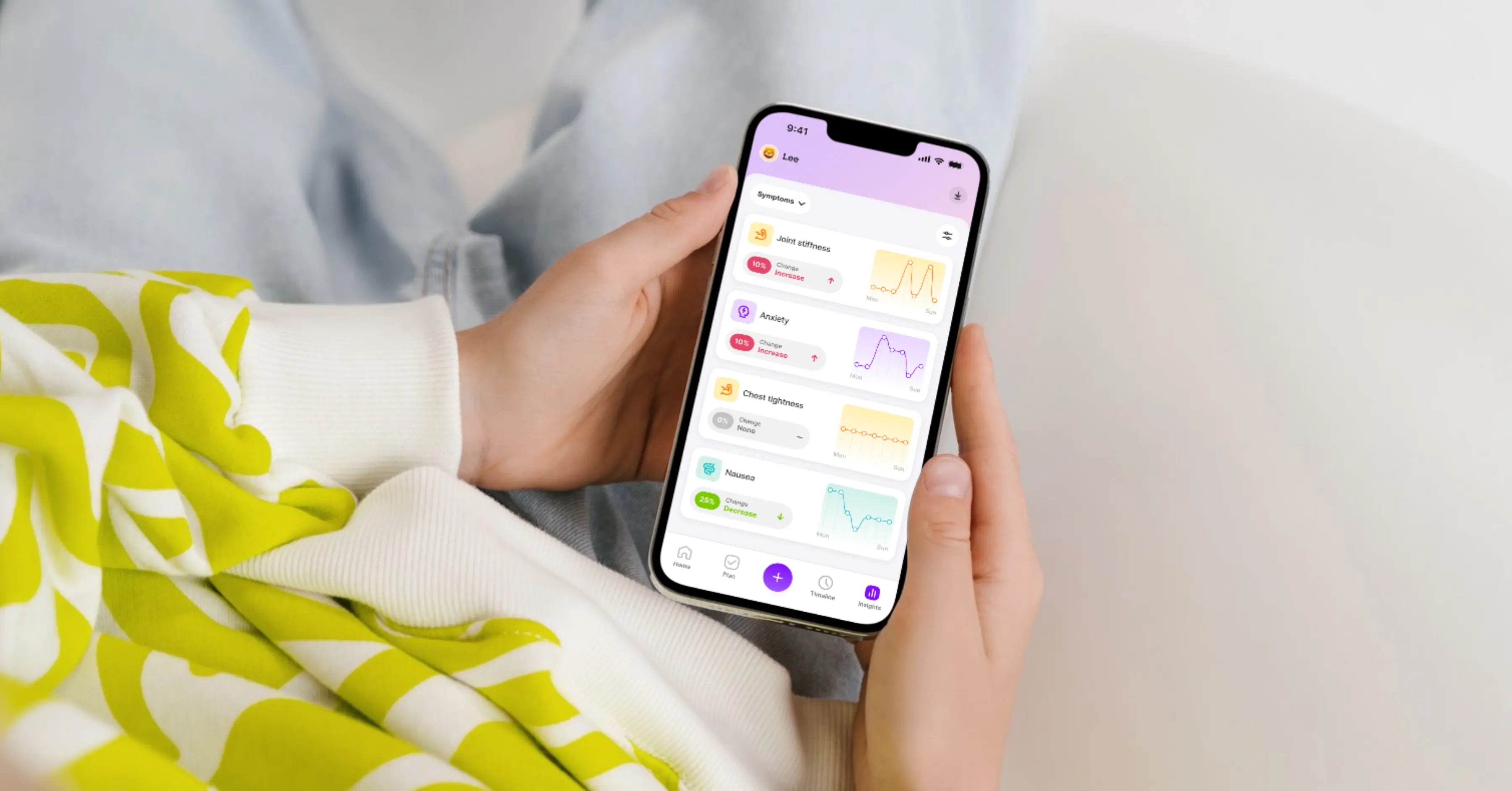
Act 1 for Human Health was about measurement: helping people capture their health story, understand what is working and advocate for better care. Today, more than 200,000 people across the United States, United Kingdom and Canada have logged over 20 million health actions on the platform to better understand their own health
.webp)
Act 2 is about intelligence: connecting those lived experiences back to science. We are building one of the world’s largest patient-led observational studies into chronic disease. Observational data cannot prove causation, but it can reveal real-world patterns that guide discovery and generate hypotheses for future clinical and molecular research.
The NIH All of Us program and the UK Biobank have already shown the power of longitudinal data to uncover new associations [15][16]. Those projects focus largely on genomics and clinical data. Our work extends that vision into everyday life, where health actually happens.
Every record in Human Health belongs to the person who created it. With their consent, their experiences can help build a faster, more responsive kind of science.
As part of our next act, we are also building HumanEvidence™, a research platform that enables scientists and research partners to capture continuous, patient-reported information in real-world settings. It aligns with the FDA’s 2024 Real-World Evidence Guidances, the EMA’s 2024 guidance on patient-experience data, and bridges the gap between clinical trials and lived experience [17][18].
Together, these layers of patient insight and scientific validation bring us closer to a healthcare system that finally learns from every life it touches.
For the Future of Human Health

We have raised $8.5 million AUD to take the next step in building the missing layer of medicine. The round brought in new international investors, LocalGlobe (UK), alongside continued support from Airtree Ventures, Skip Capital, and David Shein, with participation from Scale Investors, Aliavia, and new angels including Eric Salama (former CEO of Kantar), Arvind Rajan (founder of Cricket Health), and Kristy Chong (founder of Modibodi). All share our belief that healthcare should be more human, more precise, and more inclusive.
In an era where capital often chases quick wins and short-term hype, we are deeply grateful to our investors and team for having conviction in a mission that demands time, patience, and persistence. They see what we see: that real transformation in healthcare starts with building the right foundation.
We built this company because we have lived it: in our families, in our own health and now through the people who use Human Health every day. Each story from someone who finally feels understood strengthens our belief that this long game is worth playing.
What began as a personal mission has become a collective one, powered by a team and community determined to make healthcare more human.
From the Front Lines of Lived Experience
Every day, people remind us why we are building Human Health:
“This app makes a chronically ill person’s life so much easier.” — AF (2025)
“It just feels so inclusive and actually peaceful.” — D (2025)
“A major help and a good reminder that I’m not just my illness.” — RC (2025)
“It’s helped me better advocate for my health and treatments.” — KW (2025)
“It doesn’t make daily tracking feel like a chore.” — FM (2025)
These voices capture what drives us. The future of healthcare will not be written in laboratories alone, but in the daily lives of the people living it, one story, one pattern, one insight at a time.
We are doing this for human health, in every sense of the word.
— Georgia Vidler & Kate Lambridis
Co-Founders, Human Health
Sources
- World Health Organization. Noncommunicable Diseases Fact Sheet, updated 2025.
- Centers for Disease Control and Prevention. Chronic Diseases in America Overview.
- Australian Institute of Health and Welfare. Chronic Conditions and Multimorbidity 2022.
- The BMJ (2023). “AI in medicine: preparing for the future while preserving care.”
- JAMA (2019). “The Review of Systems, the Electronic Health Record, and Billing.”
- BMC Health Services Research (2025). “The influence of electronic health record design on usability and patient safety.”
- Nature (2022). “AlphaFold reveals structures for most proteins known to science.”
- Insilico Medicine press releases and STAT coverage (2023). “INS018_055 enters clinical trials for IPF after <4 years of development.”
- npj Digital Medicine (2024). “AI for medical imaging: current state and future directions.”
- OpenAI (2025). “Why language models hallucinate.”
- Stanford HAI (2023). “Hallucinating Law: Legal mistakes with LLMs are pervasive.”
- MedHallu Benchmark (2025 preprint). “Hallucinations in responses to healthcare queries by LLMs.”
- Nature (2025). “Clinical trials keep leaving women out.”
- International Journal for Equity in Health (2025). “Under-representation of ethnic-minority participants in health research: an umbrella review.”
- NIH All of Us Research Program overview.
- UK Biobank overview.
- US FDA. Final Guidance: Considerations for Use of RWD/RWE to Support Regulatory Decision-Making (2023) and 2024 updates.
- European Medicines Agency. Reflection Paper on Patient Experience Data and Real-World Evidence, 2024.
This is a div block with a Webflow interaction that will be triggered when the heading is in the view.













.webp)
.png)

