New Treatments for Fibromyalgia in 2025: What’s Working
September 2, 2025

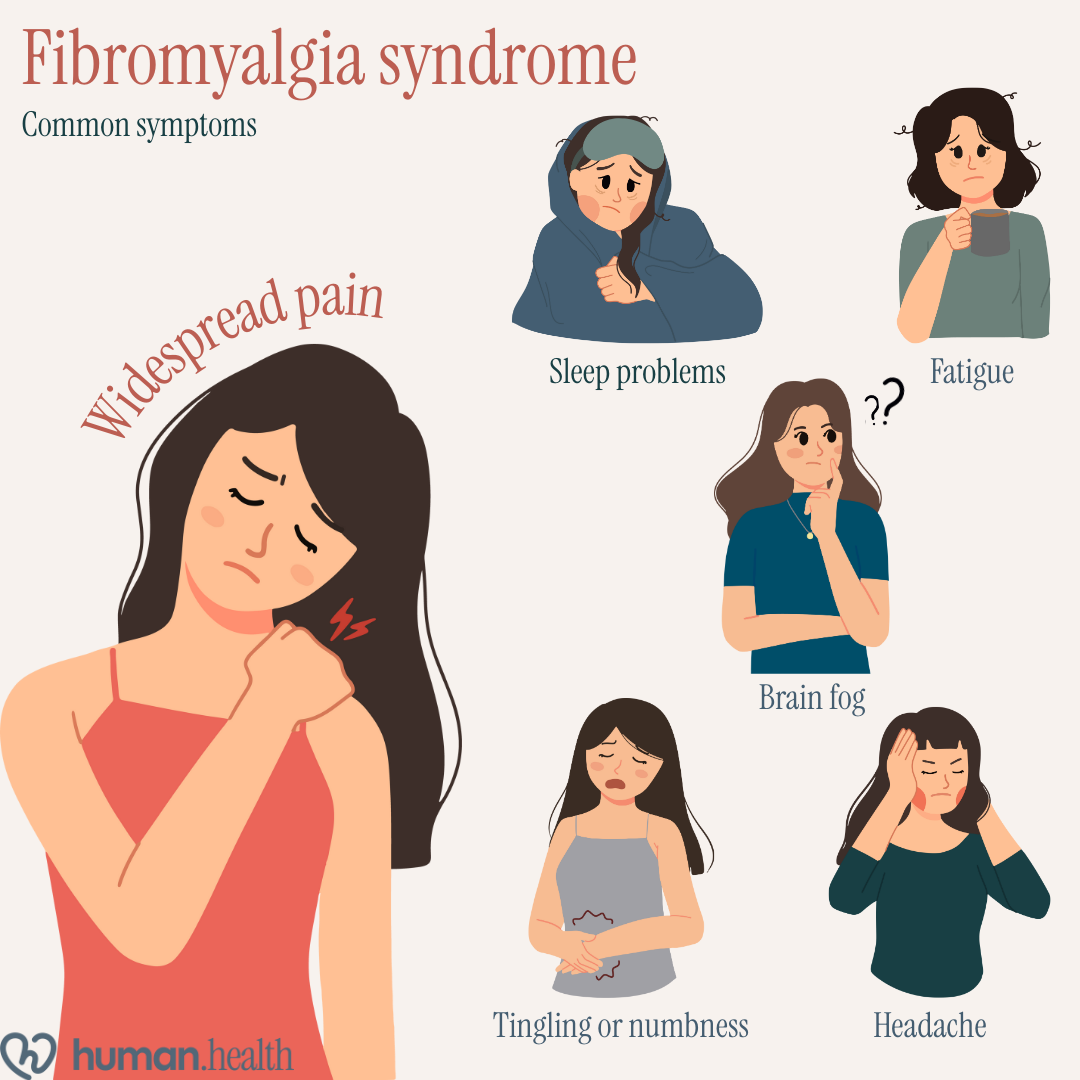
- Fibromyalgia is a chronic pain condition, with common symptoms including widespread musculoskeletal pain, sleep problems, fatigue, brain fog, headaches, and changes in sensation.
- The FDA has fast-tracked approval for a new fibromyalgia treatment, Tonmya, which may impact pain perception, muscle relaxation, and sleep pathways to relieve symptoms of fibromyalgia.
- Emerging research suggests that low-dose naltrexone, cannabinoids, and dietary changes and supplements could have positive effects for people with fibromyalgia.
- Current standards of care for fibromyalgia include pregabalin, and antidepressants such as duloxetine and milnacipran. Supportive care therapies such as exercise and psychotherapy are also recommended
- Human Health is a free app that can help you manage fibromyalgia symptoms and treatments.
Disclaimer: Human Health is not recommending any specific medical treatment for any particular symptom, nor providing any other medical advice. Always seek the advice of your doctor regarding any medical concern.
Descriptions of fibromyalgia in medical literature can be traced back to the early 1900s, and yet treatment options have been limited, until recently.
Since its official definition in the 1980's, fibromyalgia research has progressed, and clinical trials have identified some treatments that can help people with fibromyalgia manage their condition.
In this post, we'll explore new and upcoming treatment options for fibromyalgia.
What is fibromyalgia?
Fibromyalgia syndrome is a chronic pain condition that affects the central nervous system. People with fibromyalgia experience severe, widespread pain, often described as "hurting all over". Other major symptoms include poor sleep quality, and fatigue. Mood problems, brain fog, headaches, and sensations like tingling or numbness are also commonly experienced. and persistent low energy in woman, which can make daily life especially challenging. Mood problems, brain fog, headaches, and sensations like tingling or numbness are also commonly experienced.
It affects more females than males, and is often misdiagnosed or under-diagnosed. Sometimes these symptoms are confused with conditions such as polymyalgia rheumatica vs fibromyalgia, since they share overlapping signs but actually require very different treatment approaches.
New evidence-backed fibromyalgia treatment
Tonmya (cyclobenzaprine HCl)
Tonmya was "granted Fast Track FDA-approval" for the treatment of adults with fibromyalgia syndrome in August 2025, due to the unmet need for new pharmaceutical therapies for fibromyalgia since the last drug approval in 2009. It's produced by "Tonix pharmaceuticals."
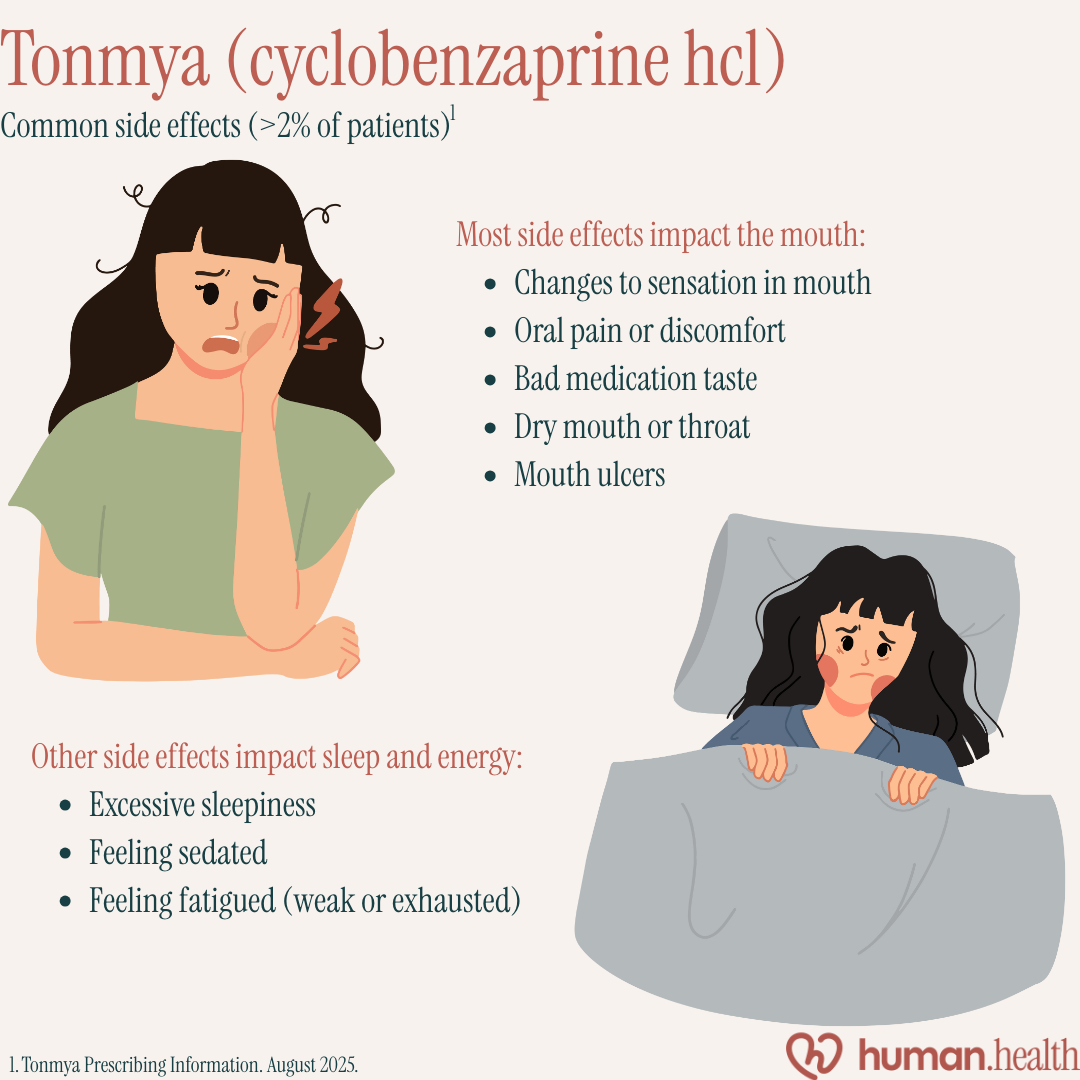
The tablet is taken at bedtime, and it dissolves under the tongue to rapidly absorb the medicine, called “cyclobenzaprine hydrochloride,” into the blood.
The way Tonmya works is not clear, but "laboratory studies" found that it blocks the activity of certain pathways in the brain that impact pain, sleep and “muscle relaxation”. Blocking these pathways may help relieve symptoms of fibromyalgia such as widespread musculoskeletal pain, and sleep problems.
In "clinical trials", Tonmya was shown to be effective at reducing daily pain scores over 14 weeks compared to placebo. In most clinical trials, Tonmya demonstrated an acceptable safety profile, although "one study" did show an unprecedented increase in side effects and discontinuations.
However, this pattern was consistent across patients taking Tonmya and those taking a placebo, and as such may have been due to factors outside of the study.
Here's a summary of common Tonmya side effects:
New research for fibromyalgia treatments
Although Tonmya is the only new pharmacological treatment approved for the treatment of fibromyalgia in recent years, scientists and clinicians are continuing research efforts to find safe and effective treatment options for people with fibromyalgia.
We've compiled a list of treatments that are being investigated for the treatment of fibromyalgia below.
Please note that these treatments are still experimental and have not been clinically proven to be effective in the treatment of fibromyalgia. Please consult with a healthcare professional before starting any new treatment.
Low-dose naltrexone
“Naltrexone” is a medication that blocks the activity of opioids, which modulate the effects of pain by acting on the brain and spinal cord. This sounds counterintuitive for fibromyalgia treatment–wouldn’t we want to provide relief by allowing opioids to exert their natural effects? The answer is yes, but by doing things a little differently.
At low doses, naltrexone only blocks opioid activity for short periods of time. In response, the body ramps up production of certain chemicals like opioids, to counteract this blockade. As a result, patients may experience a decrease in pain due to the increase in production of natural painkillers. “Inflammation of the nervous system” has also been thought to play a role in the development of fibromyalgia, and some laboratory research suggests that low-dose naltrexone may reduce this inflammation.
“Early, small studies” in patients with fibromyalgia have reported low-dose naltrexone to be an effective treatment that reduces pain in fibromyalgia, although confirmation of these results in larger clinical trials and with comparison to control groups is required.
Researchers in these studies deemed the treatment as safe and tolerable, with common side effects including vivid dreams, and gastrointestinal symptoms such as nausea, abdominal pain, constipation, and diarrhea.
{{inline-cta-1}}
Cannabinoids
Cannabinoids are substances derived from the cannabis plant, and the cannabinoids most often used therapeutically are tetrahydrocannabinol (THC) and cannabidiol, although many others exist. Cannabinoids work to relieve pain by acting on certain receptors in the brain that are involved in the perception of pain. They may also have other functions that could be beneficial to people with fibromyalgia, such as impacting sleep, mood, and muscle relaxation.
The use of medicinal cannabis for chronic pain syndromes including fibromyalgia is a rapidly progressing area of research, especially with the 2018 Fast Track FDA approval of CBD-based products for the treatment of certain types of epilepsy, a condition which also affects the nervous system.
Some clinical trials have reported promising effects of cannabinoids for people with fibromyalgia, such as decreased pain scores, improved sleep, and increased quality of life scores. Since many of the completed studies have been in small populations, further evidence will be required before cannabinoids can be proven to be as safe and effective as the currently approved treatments for fibromyalgia.
Nutraceuticals and diet changes
‘Nutraceuticals’ are nutrients which may have some therapeutic effects. For example, palmitoylethanolamide (PEA) is found naturally in the body, as well as in foods like egg yolk and peanuts, and is suggested to have anti-inflammatory properties. Acetyl-L-carnitine (ALC) can be found in plants and animals, and may have a variety of effects on the brain and nervous system, including improving cognitive functions and peripheral nerve damage.
In some small studies, PEA and ALC were found to have positive effects for people with fibromyalgia when taken in addition to their usual treatments.
Diets such as the Low-FODMAP diet, designed to avoid inflammatory foods, and versions of the Mediterranean diet, which is rich in foods that fight inflammation, may also have a positive impact when implemented alongside traditional fibromyalgia treatments.
Current standard of treatment for fibromyalgia
In 2025, there are 3 medications that have been FDA-approved for the treatment of fibromyalgia. They were approved based on the results from large clinical trials, showing significant improvements in fibromyalgia symptoms and acceptable safety profiles.
Pregabalin (Lyrica)
The first FDA-approved drug for fibromyalgia in 2007, pregabalin mimics a natural chemical in the brain called GABA. The role of GABA is to decrease the activation of cells in the brain, and pregabalin works in a similar way. This may lead to blocking the perception of musculoskeletal pain in fibromyalgia patients.
Duloxetine (Cymbalta)
This drug was approved in 2008, and acts as a serotonin and norepinephrine reuptake inhibitor (SNRI). This means it increases the amount of serotonin and norepinephrine available in the brain, which may reduce the transmission of pain signals from the brain, and provide relief from musculoskeletal pain.
Milnacipran (Savella)
Milnacipran is another SNRI that was approved for use for fibromyalgia in 2009. It works similarly to duloxetine.
Non-pharmacological treatments for fibromyalgia
According to clinical guidelines for the treatment of fibromyalgia, there are a number of other therapeutic measures patients can take which may help relieve their symptoms, outside of medicinal treatment.
Psychotherapy
Fibromyalgia affects the central nervous system, which includes the brain and spinal cord. Helping patients to retrain their brains using cognitive behavioral therapy (CBT) may provide supportive care for fibromyalgia patients. Research shows a strong association between CBT and positive improvements in areas of sleep, mood, and pain.
Exercise
Physical activity of various formats may help manage symptoms and improve quality of life for fibromyalgia patients. Examples include yoga, aquatherapy, and Eastern practices such as Qi Gong and Tai Chi.
How technology can help you manage fibromyalgia
New technologies are always being developed that can make life for people with chronic diseases like fibromyalgia easier.
Human Health is a free app designed to take the stress out of managing complex health conditions, putting you back in control of your health. Here’s how Human can help you:
Symptom tracking
Human’s extensive symptom database allows you to track the symptoms that matter to you, such as musculoskeletal pain, fatigue, and sleep problems, and the impact they’re having on your day. The app’s comprehensive Insights page helps you understand how your symptoms are changing over time, and identify patterns like triggers which could be affecting your health, like stress, illness, or weather.
Learn more about Human’s symptom tracking feature in this post.
Treatment plan
The Plan page provides you with a daily summary of which treatments you need to complete and when. With notifications, you can get reminded when it’s time to complete them, so you never miss a treatment again. An integrated calendar makes it easy to see which treatments you’ve taken, and treatments in your Plan are highly customizable, making adjustments and changes simpler than ever.
For more insights, check out our in-depth guide on treatment tracking.
Health reports
Downloadable health reports package your health journey into a concise summary, highlighting important information like the symptoms that are impacting you most, your current treatment plan, and your medical conditions. Reports take the stress out of clinic visits, empowering you to communicate with your healthcare team about what’s working and what needs to change.
Notes as a journal
With Human’s Notes feature, you can add entries about what’s going on in your life, as it happens. These entries could help support you by providing a space to write down your thoughts and feelings, or add extra info about that new symptom you’ve been having.
Explore our mental health journal for additional details.
Appointment reminders
Fibromyalgia management often involves input from a number of different practitioners, including physiotherapists, psychologists or psychiatrists, rheumatologists, pain specialists, and more. Now you can add appointments into Human and get reminders when they’re coming up. Other features include linking treatment tasks to their relevant appointment, reminders for recurring appointments, and a schedule view so you can see all your appointments in one place.
{{inline-cta-2}}
References
- Tonix Pharmaceuticals Announces FDA Approval of Tonmya™ (cyclobenzaprine HCl sublingual tablets) for the Treatment of Fibromyalgia. Press Release. August 15, 2025.
- Tonmya Prescribing Information, August 2025.
- Lederman S, Arnold LM, Vaughn B, Kelley M, Sullivan GM. Efficacy and Safety of Sublingual Cyclobenzaprine for the Treatment of Fibromyalgia: Results From a Randomized, Double‐Blind, Placebo‐Controlled Trial. Arthritis Care & Research. 2023 Nov;75(11):2359-68.
- Khan I, Kahwaji CI. Cyclobenzaprine. [Updated 2023 Aug 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513362/
- Seth Lederman, Lesley M Arnold, Ben Vaughn, Jean M Engels, Mary Kelley, Gregory M Sullivan, Pain relief by targeting nonrestorative sleep in fibromyalgia: a phase 3 randomized trial of bedtime sublingual cyclobenzaprine, Pain Medicine, 2025;, pnaf089, https://doi.org/10.1093/pm/pnaf089
- Tonmya * (TNX-102 SL) in Development for the Management of Fibromyalgia. At Controversies in Fibromyalgia - Brussels. Version P0535 March 7, 2024 (Doc 1389).
- Vatvani AD, Patel P, Hariyanto TI, Yanto TA. Efficacy and safety of low-dose naltrexone for the management of fibromyalgia: a systematic review and meta-analysis of randomized controlled trials with trial sequential analysis. The Korean Journal of Pain. 2024 Oct 1;37(4):367-78.
- Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol. 2014 Apr;33(4):451-9. doi: 10.1007/s10067-014-2517-2. Epub 2014 Feb 15. PMID: 24526250; PMCID: PMC3962576.
- Aitcheson N, Lin Z, Tynan K. Low-dose naltrexone in the treatment of fibromyalgia: A systematic review and narrative synthesis. Australian Journal of General Practice. 2023 Apr;52(4):189-95.
- Fisher E, Moore RA, Fogarty AE, Finn DP, Finnerup NB, Gilron I, Haroutounian S, Krane E, Rice AS, Rowbotham M, Wallace M. Cannabinoids, cannabis, and cannabis-based medicine for pain management: a systematic review of randomised controlled trials. Pain. 2021 Jul 1;162:S45-66.
- Drugbank. Cannabidiol. Available at: https://go.drugbank.com/drugs/DB09061
- National Center for Complementary and Integrative Health. Cannabis (Marijuana) and Cannabinoids: What You Need To Know. Available at: https://www.nccih.nih.gov/health/cannabis-marijuana-and-cannabinoids-what-you-need-to-know
- Lopera V, Restrepo JC, Amariles P. Effectiveness and safety of cannabis-based products for medical use in patients with fibromyalgia syndrome: A systematic review. Exploratory Research in Clinical and Social Pharmacy. 2024 Dec 1;16:100524.
- Puri V, Nagpal M, Singh I, Singh M, Dhingra GA, Huanbutta K, Dheer D, Sharma A, Sangnim T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients. 2022 Nov 3;14(21):4637. doi: 10.3390/nu14214637. PMID: 36364899; PMCID: PMC9654660.
- Drugbank. Acetylcarnitine. Available at: https://go.drugbank.com/drugs/DB08842
- Lambiase C, Rossi A, Morganti R, Cancelli L, Grosso A, Tedeschi R, Rettura F, Mosca M, de Bortoli N, Bellini M. Adapted low-FODMAP diet in IBS patients with and without fibromyalgia: long-term adherence and outcomes. Nutrients. 2024 Oct 9;16(19):3419.
- Casini I, Ladisa V, Clemente L, Delussi M, Rostanzo E, Peparini S, Aloisi AM, de Tommaso M. A personalized Mediterranean diet improves pain and quality of life in patients with fibromyalgia. Pain and Therapy. 2024 Jun;13(3):609-20.
- Pfizer's Lyrica Receives FDA Approval for Fibromyalgia Based on Expedited Review. Press Release. June 21, 2007.
- Cross AL, Viswanath O, Sherman AL. Pregabalin. [Updated 2024 May 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470341/
- FDA Approves Cymbalta(R) for the Management of Fibromyalgia. Press Release. June 16, 2008.
- Drugbank. Duloxetine. Available at: https://go.drugbank.com/drugs/DB00476
- Forest and Cypress Announce FDA Approval of Savella(TM) for the Management of Fibromyalgia. Press Release. January 14, 2009.
- Jones EA, Asaad F, Patel N, Jain E, Abd-Elsayed A. Management of Fibromyalgia: An Update. Biomedicines. 2024 Jun 6;12(6):1266. doi: 10.3390/biomedicines12061266. PMID: 38927473; PMCID: PMC11201510.
This is a div block with a Webflow interaction that will be triggered when the heading is in the view.

Start Tracking Your Condition Inside The Human Health App!
Gain insight into how your treatment plan impacts your symptoms, and talk to your providers with confidence & be heard.
Try The Human App Today!
Human is the health app that helps people with chronic conditions track what matters, see what's working, and advocate for better care - with or without a specialist by your side.







.jpg)





.png)

